Explain How Water May Have Two Different Densities
Note that water can be supercooled and remain a liquid well below its normal freezing point. Seawater is denser than freshwater.
Density Temperature And Salinity Manoa Hawaii Edu Exploringourfluidearth
Density mass volume Mass of steel cube 468 g Volume of steel cube 60 cm3 Density.

. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. The maximum density of water occurs around 4 degrees Celsius. This behavior of the density of water explains why ice forms at the top of a body of water.
Mass - some substances are heavier than others. Temperature and salinity both affect the density of water. When it is cooled from the room temperature the liquid water tends to become increasingly dense as with another kind of substances but approximately at about 4C pure water is said to reach its maximum density.
An atom of iron is heavier than a carbon atom. Calculate the density by dividing the mass by the volume. This means that ice is slightly less dense than cold water which is why ice floats on the surface of bodies of water.
As the temperature of the water decreases the density of the water increases. The density of pure water actually is somewhat less than 1 gcm 3. This makes water more dense than oil.
PMv That is density p is equal to total mass M divided by total volume v. When water cools it causes the molecules to move closer together as they slow down. This formula can be used to determine the density of any substance.
Unlike most substances water is denser as a liquid than as a solid. You calculate its density using the formula ρ m V ρ Density kgm3 m Mass kg V Volume m3 So drop the numbers of your measurements into the equation. Molecules of nonpolar compounds such as oil and gasoline even when mixed well into water tend to separate from the water when the mixing stops.
Density is an intensive property which means. The density of a substance is not necessarily constant throughout the volume of a substance. Because of density differences between water and oil this means that they form two separate liquid layers.
Specific gravity is defined as the ratio of the density of the material to the density of water at 40C and one atmosphere of pressure which is 1000kgm3. For example cork has a density of 240 kgm 3 so it will float. When water freezes at 0C a rigid open lattice like a web of hydrogen-bonded molecules is formed.
Water is most dense at 39F and as it cools or warms from this temperature the water expands slightly. Water is the chemical substance with chemical formula H 2 O. It is this open structure that makes ice less dense than liquid water.
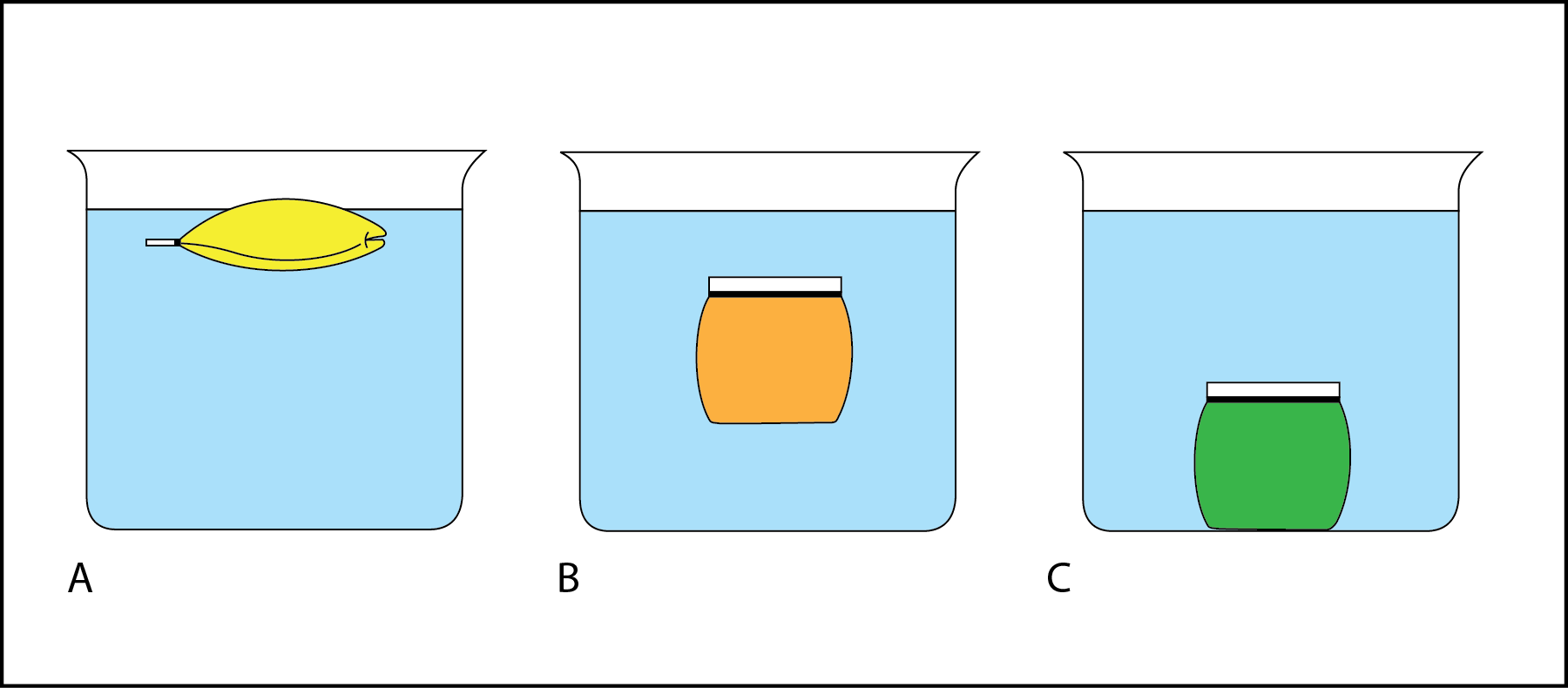
A consequence is that ice floats on water. The density of water shows why objects float. Using Water as a Density Comparison When an object is placed in water the objects relative density determines whether it floats or sinks.
Water molecules tend to hold on to each other and squeeze out nonpolar oil and gasoline. The density of water increases with decreasing temperature reaching a maximum at and then decreases as the temperature falls below. It is helpful to know the formula for density.
Topics Concepts Citizen science Teacher PLD Glossary Sign in. Density at different temperatures Water at. Density is a measurement of the mass per unit volume and it has an SI unit of kgm3.
Water molecules are made up of oxygen and hydrogen atoms bonded together. The density of water once again is a special case. The relationship between mass density and volume tells you how density measures the ratio of an objects mass to its volume.
Density is calculated according to the simple formula. Density massvolume so they would only have the same density if they also have the same mass. Plug in the values of mass and volume you determined and solve.
One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Also pure water is less dense than seawater so fresh water can float on top of salt water mixing at the interface. Temperature salinity and water density Science Learning Hub Temperature salinity and water density Add to collection Cold water is denser than warm water so it tends to sink.
Note that the density of pure water is defined to be 1 gram per cubic centimeter or gml. Describing them requires knowing the equations that lie beneath them. Common units for the measurement of density include grams g milliliters ml or grams per cubic centimeter.
Volume of the water. Sciencing_Icons_Science SCIENCE Sciencing_Icons_Biology Biology. Ice is less dense than liquid water so it floats.
30C is 09957 g cm-³ 4C is 10000 g cm-³ 0C is 09998 g cm-³ How does salinity affect water density. Density equals the mass of the object divided by its volume. Salinity temperature and depth all affect the density of seawater.
Using the equation density massvolume you can determine the density of water. From the equation above we can see that the density of a substance depends on two quantities. This slowdown of the molecules causes the water to become denser.
Specific gravity Density of material Density of water. This is why icebergs float. Also water molecules are very attracted to each other and pack very close together.
As it gets cooled further it tends to expand and becomes less dense. Summary Students use the water displacement method to find the volume of different rods that all have the same mass. This can easily be observed in a.
The comparison uses water because the density of water is 1gcm3 which was originally used to define the kilogram. Project the image Water Oxygen is heavier and smaller than carbon so a volume of water molecules is heavier than the same volume of oil molecules. Using those results the densities can be calculated using.
Objects with the same mass but different volume have different densities. An object with a higher density will sink. If the object has a lower density than water it will float to the top of the water.
This makes the density unit mass volume. ρ 5400kg 2m3 and the answer is ρ 2700kgm3 Aluminium has a density of approx 2705kgm 3 and Stainless Steel has a density of approx 7982kgm 3. A standard table lists the values for the density of liquid water.
Mass of the water.

What Is The Density Of Water Factors Experiment Temperature Scales Faqs

Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry

No comments for "Explain How Water May Have Two Different Densities"
Post a Comment